Embarking on any research study involving human participants brings with it a profound responsibility: ensuring their rights, welfare, and understanding are at the forefront. This isn’t just a legal or ethical nicety; it is the very bedrock upon which sound, credible research is built. A well-crafted inform consent document serves as a critical bridge, allowing participants to fully grasp the nature of the study, what their involvement entails, and crucially, that their participation is entirely voluntary.
Beyond just understanding the study, many research designs also incorporate randomization, a powerful tool used to minimize bias and ensure the fairness of treatment or intervention allocation. Combining these two elements into a comprehensive, clear, and easy-to-understand form can seem like a daunting task. However, having a structured approach, perhaps starting with a robust inform consent and randomization form template, can simplify the process immensely, ensuring all necessary ethical and methodological considerations are addressed thoroughly.

Why a Robust Inform Consent and Randomization Form Template is Indispensable
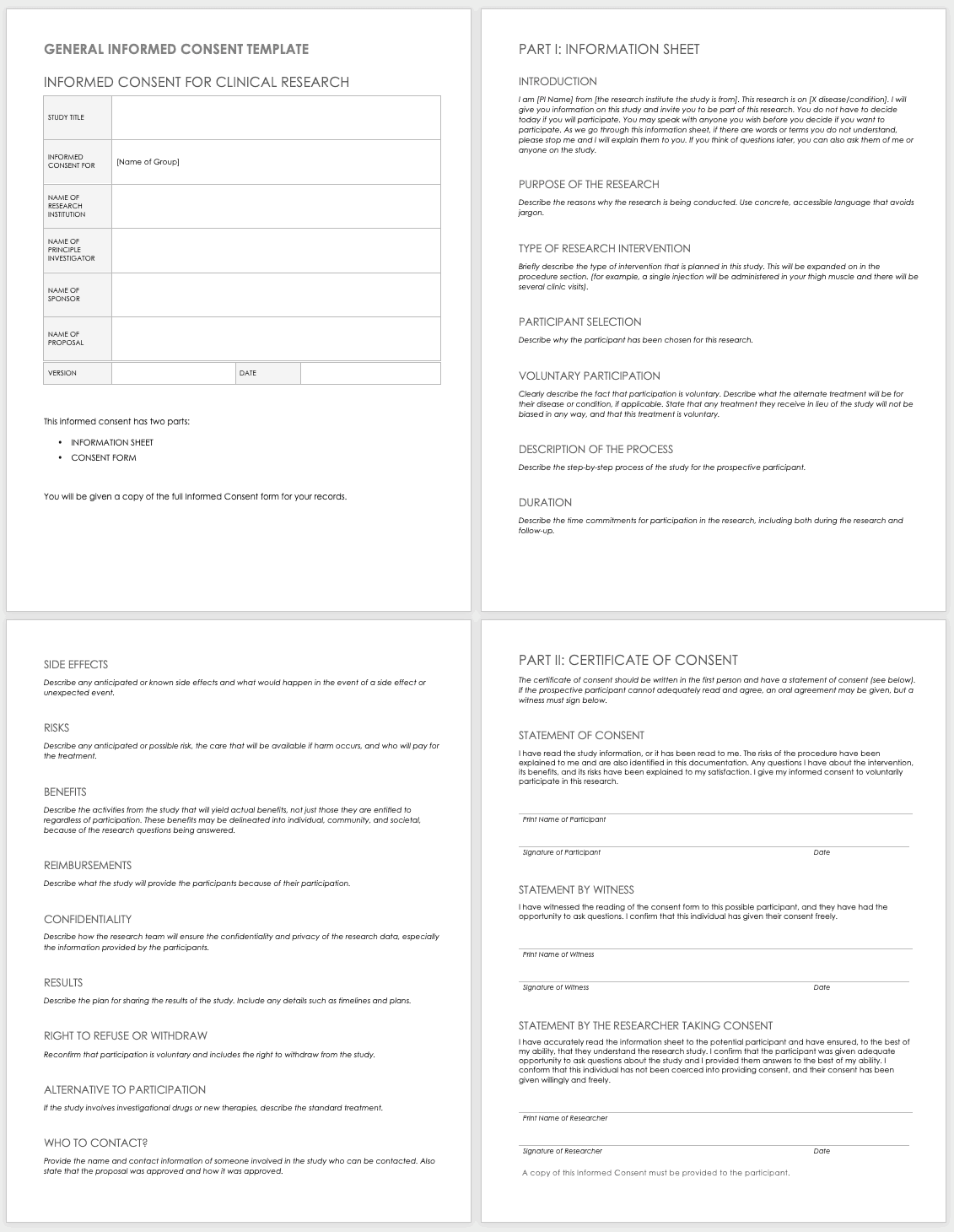
The ethical imperative of informed consent cannot be overstated. It upholds the fundamental right of individuals to make autonomous decisions about their own bodies and participation in research. A properly executed consent process means that potential participants are given all the information they need, in a language they can understand, to decide whether or not to join a study. This includes clear explanations of the study’s purpose, procedures, potential risks, anticipated benefits, and their right to withdraw at any time without penalty. Without this foundational step, research lacks ethical integrity, regardless of its scientific merit.
Randomization, on the other hand, is a cornerstone of experimental research design, particularly in clinical trials. Its primary purpose is to eliminate selection bias by ensuring that participant assignments to different study groups (e.g., treatment group vs. control group) are purely by chance. This random allocation helps to create comparable groups, meaning any observed differences in outcomes are more likely due to the intervention being studied rather than pre-existing differences between the groups. Explaining this concept clearly, without revealing the allocation, is a delicate but crucial part of the consent process.
Key Components of an Effective Template
A well-designed template helps ensure that no vital information is overlooked, providing a consistent framework for all participant interactions. It acts as a checklist, ensuring compliance with ethical guidelines and regulatory requirements. Key elements typically include:
- A clear statement of the study’s purpose and its duration.
- A detailed description of all procedures participants will undergo.
- An honest assessment of foreseeable risks or discomforts and potential benefits.
- Assurances regarding the confidentiality and privacy of participant data.
- Explicit affirmation that participation is voluntary and the participant’s right to withdraw.
- An explanation of the randomization process, including the odds of being assigned to a particular group.
- Contact information for the research team and institutional review board (IRB) for questions or concerns.
- A section for participant signatures and the date, acknowledging their understanding and consent.
The practical benefits for researchers are also substantial. Using a standardized inform consent and randomization form template streamlines the consent process, saves time, reduces the potential for errors or omissions, and provides a clear audit trail for regulatory bodies. It helps ensure that all participants receive the same comprehensive information, promoting consistency across the research project.
Crafting Your Own Inform Consent and Randomization Form Template: Best Practices
When developing your specific form, always prioritize clarity and simplicity. Avoid scientific jargon or overly complex sentences. Imagine explaining the study to a friend or family member who has no prior knowledge of your field. This approach helps ensure that the document is truly understandable to a diverse range of participants. Remember, the goal is not just to get a signature, but to ensure genuine comprehension and voluntary participation based on that understanding.
For the randomization aspect, it is essential to explain that randomization will occur and why it is used (to ensure fairness and unbiased results), without revealing how a specific participant will be assigned or what their assignment will be. For example, you might state that "participants will be randomly assigned to one of two groups, much like flipping a coin, to ensure the study is fair." This helps manage expectations and reinforces the scientific rigor of your methodology.
Some key practices to consider when tailoring your template include:
- Tailor to your specific study: While a template provides a base, customize every section to reflect the unique aspects of your research.
- Test clarity with a lay audience: Have someone outside your field read the draft and provide feedback on clarity and comprehensibility.
- Include version control: Clearly label your forms with version numbers and dates, especially if revisions are made during the study.
- Seek IRB/Ethics Committee approval early: Engage with your institutional review board or ethics committee during the drafting phase to incorporate their feedback.
- Train staff on the consent process: Ensure all personnel involved in obtaining consent are thoroughly trained not just on the form, but on the process of informed consent, which involves open communication and answering questions.
Remember, the form is just one part of the consent process. It should be accompanied by ample opportunity for potential participants to ask questions, have those questions answered thoroughly, and take their time to consider their decision in a comfortable, pressure-free environment. This interactive discussion is as vital as the written document itself. Adapting your inform consent and randomization form template to different study types, participant demographics, and cultural contexts will further enhance its effectiveness and ethical compliance, ensuring your research is both scientifically sound and ethically robust.
Creating a comprehensive and clear consent form is a foundational step in any ethically sound research endeavor. It empowers individuals to make informed choices about their involvement, while also providing a framework for robust methodological practices like randomization. Focusing on transparency and participant understanding builds trust, which is invaluable for the success and integrity of any study. The diligent preparation of these vital documents ultimately contributes to research that is not only scientifically rigorous but also deeply respectful of human dignity and rights.