Starting a new research study can feel like a massive undertaking, right? There’s so much to organize, from the scientific protocol to the ethical considerations that protect the participants. One document that truly sits at the heart of ethical research is the combined informed consent and randomization form. It’s more than just a piece of paper; it’s a vital communication tool ensuring participants fully understand what they’re signing up for, and how their involvement contributes to scientific discovery.
Crafting such a document from scratch can be time-consuming and complex, especially when you need to cover all the legal, ethical, and practical bases. That’s why having a well-structured informed consent and randomization form template can be an absolute lifesaver for researchers, ethics committees, and study coordinators alike. It provides a solid foundation, ensuring all critical elements are included while offering the flexibility to tailor the specifics to your unique study.
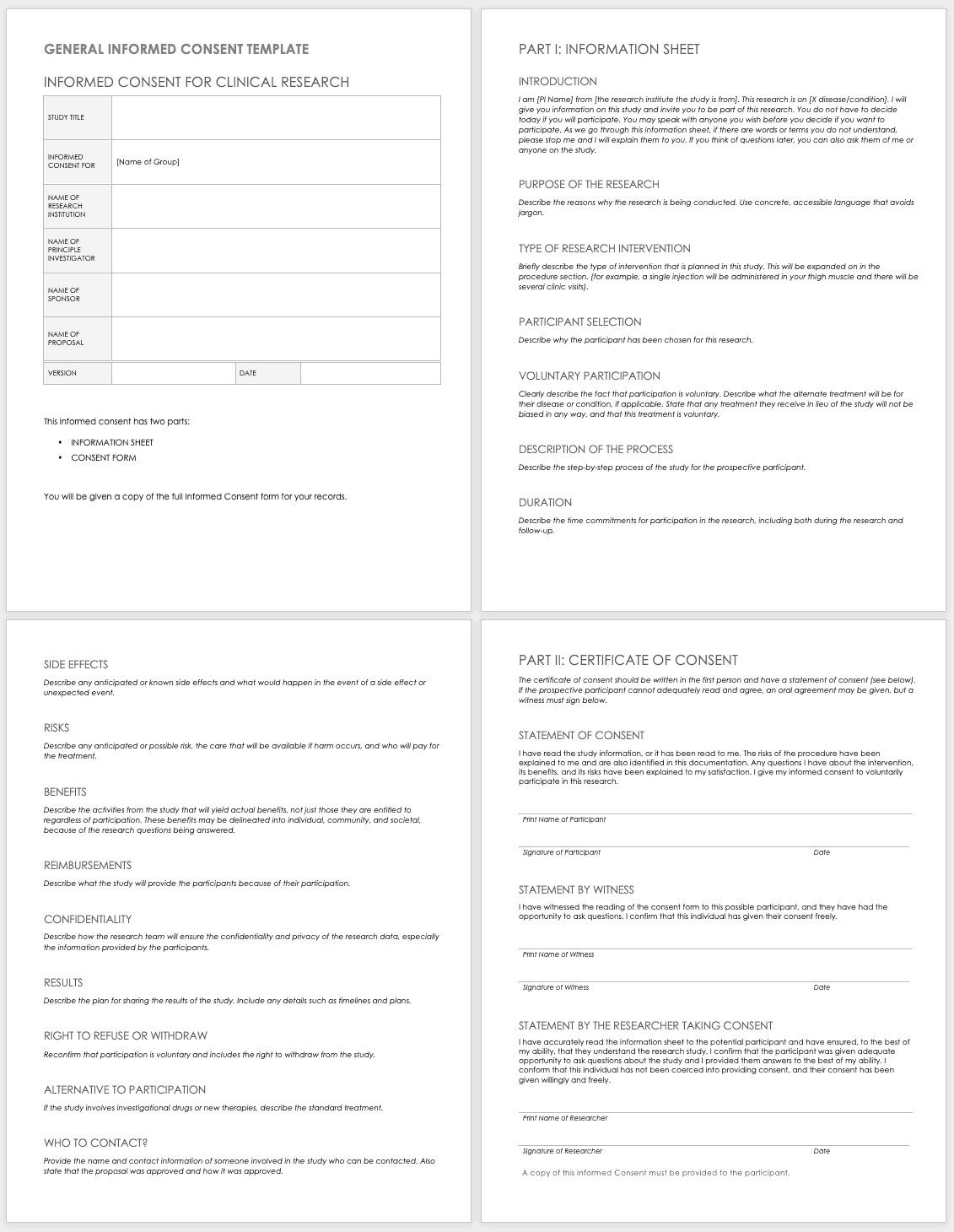
Understanding the Essentials of an Informed Consent and Randomization Form Template
In any clinical trial or research study involving human participants, transparency and ethical conduct are paramount. The informed consent process is the cornerstone of this ethical framework, ensuring that individuals volunteer to participate based on a comprehensive understanding of the study’s purpose, procedures, potential risks, and benefits. It’s not just a legal requirement; it’s about respecting individual autonomy and building trust between researchers and participants.
The “informed consent” part of the form delves deeply into what participation entails. This includes a clear explanation of the study’s objectives, what procedures a participant will undergo, any discomforts or side effects they might experience, and the potential advantages, if any, they could gain. It also clarifies confidentiality measures and provides contact information for any questions or concerns, reinforcing the participant’s right to withdraw at any time without penalty.

Equally crucial for many studies is the “randomization” component. Randomization is a scientific method used to assign participants to different study groups (like a treatment group or a placebo group) purely by chance. This process is vital because it helps ensure that the groups are comparable in terms of known and unknown factors, reducing bias and making the study’s results more reliable and generalizable. Explaining randomization clearly, without revealing the specific arm to which a participant will be assigned, is key to maintaining the study’s integrity and participant trust.
Key Components of a Robust Template
When you’re putting together your own form, thinking about an effective informed consent and randomization form template, there are several vital sections you simply cannot overlook. Each part plays a crucial role in providing clarity and ensuring compliance. Here are some of the elements you should definitely include:
- Study Title and Purpose: Clearly state what the research is about in simple terms.
- Participant Eligibility: Briefly outline who can and cannot join the study.
- Procedures and Schedule: Detail exactly what will happen at each visit or step, including the time commitment.
- Risks and Discomforts: Be upfront about any potential negative effects, no matter how minor.
- Benefits of Participation: Explain any direct or indirect benefits, acknowledging that direct benefits might not apply to all participants.
- Confidentiality: Describe how personal information will be protected and used.
- Voluntary Participation and Withdrawal: Emphasize that participation is completely voluntary and participants can leave at any time without repercussions.
- Contact Information: Provide names and numbers for participants to ask questions or report concerns.
- Randomization Explanation: Explain what randomization means, why it’s used, and that participants will be assigned to a group by chance.
- Signature Lines: Spaces for the participant (or their legally authorized representative) and the researcher to sign and date, indicating informed agreement.
Tailoring and Implementing Your Form Effectively
While an informed consent and randomization form template offers a fantastic starting point, it’s rarely a one-size-fits-all solution. Every research study is unique, with its own specific procedures, risks, and participant demographics. Therefore, customizing your template to accurately reflect the nuances of your particular study is absolutely essential. This means going beyond just filling in the blanks; it involves carefully reviewing each section to ensure it precisely describes your research while remaining accessible and understandable to your target audience.
Before any participant ever sees your form, it must undergo rigorous review by an Institutional Review Board IRB or ethics committee. These committees play a critical role in safeguarding the rights and welfare of research participants. They will scrutinize your informed consent document for clarity, completeness, accuracy, and adherence to ethical guidelines and regulations. Being prepared for feedback and willing to revise your template based on their recommendations is a crucial part of the research process.
Beyond the content itself, the practical implementation of your informed consent and randomization form requires careful consideration. The consent discussion should always happen in a private setting, allowing participants ample time to ask questions and process the information without feeling rushed or pressured. Researchers responsible for obtaining consent must be highly trained, knowledgeable about the study, and skilled in communicating complex information in a compassionate and understandable way. Remember, the form is a tool to facilitate a conversation, not a replacement for it.
Furthermore, consider the language used within the template. It’s often recommended to write at an eighth-grade reading level or lower to ensure broad comprehension. Avoid overly technical jargon, and if medical or scientific terms must be used, provide clear and simple explanations. Using clear, concise, and participant-centric language fosters better understanding and truly empowers individuals to make an informed decision about their participation. Providing a copy of the signed form to the participant is also a best practice, giving them a reference point if they have questions later.
Ultimately, a meticulously crafted informed consent and randomization document is a testament to ethical research practices. It serves as a comprehensive guide for participants, clearly outlining their journey within the study while simultaneously upholding the scientific integrity that randomization provides. By investing time in developing or adapting a robust template, researchers can streamline their processes, ensure regulatory compliance, and most importantly, foster a foundation of trust with the individuals who contribute so much to advancing knowledge.
Embracing a well-designed informed consent and randomization form template not only simplifies the administrative burden but also elevates the quality and integrity of your research. It’s an invaluable asset for anyone involved in human subjects research, ensuring that every study begins on a solid ethical footing, safeguarding both participants and the pursuit of scientific discovery.


