Navigating the vast and complex world of medical devices can be a daunting task for any healthcare institution. From the smallest diagnostic tool to the most advanced surgical equipment, each device plays a critical role in patient care, safety, and operational efficiency. Deciding which devices to acquire isn’t just about the initial cost; it involves a deep dive into functionality, safety, compliance, usability, and long-term value. A haphazard approach can lead to costly mistakes, compromised patient outcomes, and regulatory headaches.
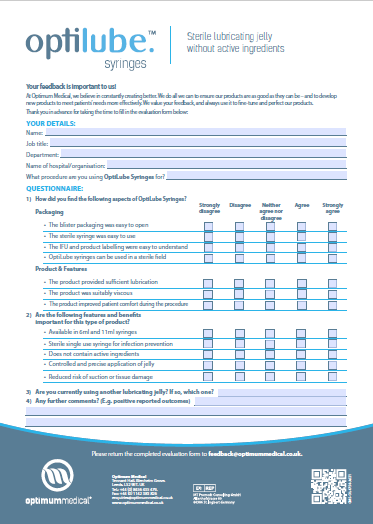
This is precisely where a well-designed medical device evaluation form template becomes an indispensable tool. It provides a structured, systematic way to assess potential new devices or re-evaluate existing ones, ensuring that every crucial aspect is considered before a decision is made. By standardizing the evaluation process, healthcare providers can make informed choices, reduce risks, and ultimately enhance the quality of care they deliver.
Why a Structured Medical Device Evaluation is Crucial
The landscape of medical technology is constantly evolving, bringing forth innovations that promise to improve diagnostics, treatments, and patient recovery. However, with these advancements come inherent risks. Every medical device, no matter how simple, carries a potential for malfunction, misuse, or adverse events if not properly vetted and implemented. A structured evaluation process acts as a vital gatekeeper, minimizing these risks and maximizing the benefits for patients and staff alike. It moves beyond subjective opinions, providing objective data that supports procurement decisions.
Such a formal process ensures that due diligence is performed on every aspect of a device, from its technical specifications to its long-term economic implications. Without a standardized approach, institutions risk inconsistent evaluations, leading to the adoption of devices that may not fully meet clinical needs, integrate well with existing systems, or offer the best value for money. This can result in unnecessary expenditure, operational inefficiencies, and, most importantly, potential compromises to patient safety and staff workflow.

The beauty of using a medical device evaluation form template lies in its ability to streamline this often-complex process. It provides a consistent framework, ensuring that all relevant departments and stakeholders – clinical staff, biomedical engineers, purchasing, IT, and risk management – contribute their expertise in a coordinated manner. This collaborative approach fosters comprehensive assessments, reduces oversights, and accelerates the decision-making cycle, all while maintaining high standards of scrutiny.
Ultimately, a robust evaluation process, underpinned by a comprehensive medical device evaluation form template, translates directly into improved patient care and operational excellence. It helps identify devices that are not only clinically effective but also safe, user-friendly, cost-efficient, and compliant with all relevant regulations. It’s an investment in quality, safety, and smart resource allocation for any healthcare facility.
Key Elements Your Evaluation Form Should Include
- Device Identification and Purpose: Clearly state the device name, model, manufacturer, and its unique identifier (UDI). Detail its intended use and the specific clinical problem it aims to solve.
- Performance and Efficacy: Assess how well the device performs its intended function. This includes looking at clinical trial data, independent studies, and user feedback regarding its effectiveness, reliability, and accuracy.
- Safety Features and Risk Assessment: Evaluate built-in safety mechanisms, potential failure modes, and documented adverse events. Consider compliance with safety standards like IEC 60601 and any specific regulatory body requirements (e.g., FDA, CE Mark).
- Usability and Human Factors: How easy is the device to operate, clean, and maintain? Consider the learning curve for staff, ergonomic design, alarm management, and integration into existing workflows.
- Cost Analysis: Beyond the purchase price, evaluate total cost of ownership including consumables, maintenance contracts, training costs, and potential for energy consumption.
- Vendor Support and Training: Assess the manufacturer’s reputation, responsiveness, and the quality of training and ongoing support they offer for the device.
- Regulatory Compliance and Documentation: Verify that the device has all necessary regulatory approvals and that required documentation (IFU, manuals, service guides) is readily available and clear.
- Compatibility and Integration: Determine if the device is compatible with existing IT infrastructure, electronic health records (EHRs), and other medical equipment in your facility.
- Post-Market Surveillance Considerations: Plan for ongoing monitoring, maintenance schedules, and potential recalls or software updates once the device is in use.
Implementing Your Medical Device Evaluation Form Template for Success
Having a meticulously designed medical device evaluation form template is an excellent start, but its true value is realized through effective implementation. It’s not merely a document to be filled out; it’s a living tool that guides a comprehensive, multi-disciplinary assessment process. Successful implementation requires clear guidelines, dedicated resources, and a commitment from all involved parties to follow the structured approach outlined in the template. This means establishing a clear workflow, defining roles and responsibilities, and ensuring that everyone understands the purpose and benefits of a thorough evaluation.
One of the most critical aspects of implementation is fostering a collaborative environment. Medical device evaluation is rarely the sole responsibility of one department. Clinical users, biomedical engineers, purchasing managers, IT specialists, infection control, and even financial departments all bring unique perspectives and expertise. The template should facilitate their input, ensuring that all relevant viewpoints are captured and considered before a final decision is made. Training sessions on how to effectively use the template and interpret its sections can significantly improve the quality and consistency of evaluations.
Furthermore, recognize that the evaluation process is often iterative. Initial assessments might raise questions that require further investigation, pilot testing, or direct interaction with the vendor. The template should be flexible enough to accommodate these deeper dives while maintaining a clear record of all findings. It also serves as an excellent reference point for future re-evaluations or comparisons when newer models or alternative devices become available, creating a valuable historical database of device performance and suitability.
To truly maximize the benefits of your evaluation template, consider integrating it into your broader quality management system. Regular review and updates of the template itself are also crucial, reflecting changes in regulatory requirements, technological advancements, or internal organizational needs. By treating the medical device evaluation form template as a dynamic tool for continuous improvement, healthcare organizations can ensure they consistently make the best possible decisions regarding their vital medical equipment.
A diligent and systematic approach to evaluating medical devices, powered by a comprehensive template, significantly strengthens a healthcare institution’s ability to deliver exceptional care. It moves beyond anecdotal evidence, providing a robust framework for informed decision-making that prioritizes patient safety, clinical efficacy, and operational efficiency. This level of scrutiny not only safeguards patients and staff but also optimizes resource allocation, ensuring that investments in technology yield the best possible returns.
By standardizing assessments and ensuring all critical aspects are considered, organizations can minimize risks, comply with regulatory requirements, and adapt to the ever-evolving landscape of medical innovation with confidence. This strategic foresight fosters a culture of excellence and continuous improvement, ultimately enhancing the overall quality and reliability of healthcare services provided.


